Mobility at the surface of amorphous material plays an important role in processes such as crystallization, sintering, corrosion, and catalysis. The knowledge of near-surface mobility provides significant information concerning the understanding of the surface crystal growth processes which are important for the preparation, processing, and utilization of amorphous materials. The near-surface mobility can be described by near-surface viscosity or by surface diffusion coefficient.
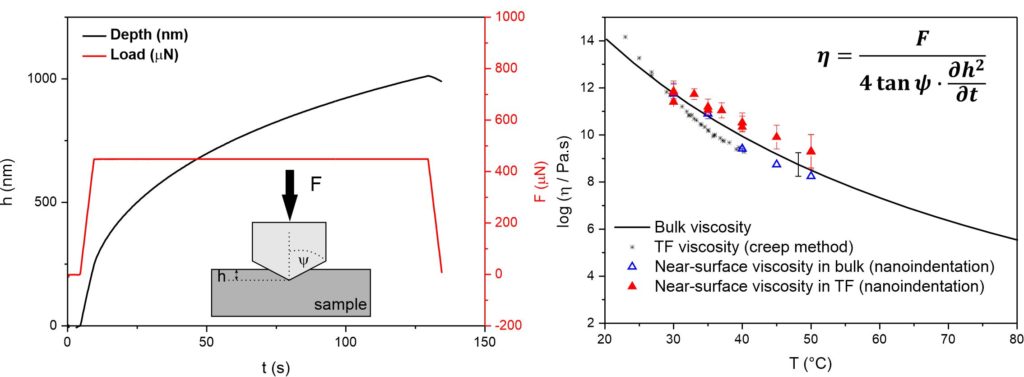
A powerful method to study near-surface viscosity in amorphous materials is nanoindentation. The method is similar to a penetration method usually used to study viscosity in bulk materials using a thermomechanical analyzer. An indenter of a defined shape is impressed into the sample under a constant load and viscosity data are obtained from the steady penetration rate. The nanoindentation is using a very small load resulting in very small penetration depths as is shown in Fig. 1. Therefore, nanoindentation provides a powerful tool to study near-surface viscosity, especially in the systems of thin films where only small depths can be measured to avoid an influence of the substrate.
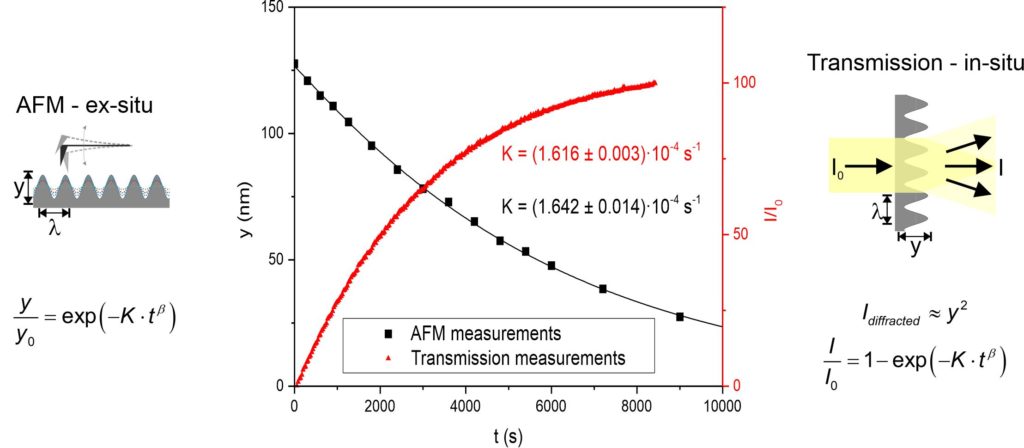
Another way to study surface mobility is by following a flattening process of defined structure embossed into the surface of amorphous material. The most used experimental setting is based on the measurements of the time change in the amplitude of a single period sinusoidal grating as is shown in Fig. 2. The amplitude change can be followed directly by measurement of transmitted or diffracted radiation, or ex-situ by atomic force microscopy (AFM) or scanning electron microscopy. The rate constant (also decay constant, K) of the flattening process can be then evaluated from the time dependence of the grating amplitude. The obtained decay constant provides information about the mobility of the structural units on the surface covering the surface diffusion and the near-surface viscosity. Except for the study of flattening of periodic structures on the surface of amorphous materials to obtain information about surface mobility, another way to study surface diffusion is to perform experiments with non-sinusoidal surface profiles. The advantage of this method is, that many Fourier components can be simultaneously probed. An isolated protrusion or hole can be represented by a sum of its Fourier components, which after individual decay, can be summed again to construct the surface profile at a later time.
Surface structuring and further study of the flattening of the surface structures bring a great opportunity to study surface mobility in amorphous systems. Nevertheless, there is one disadvantage of these experiments, or better in the data evaluation – there is a need for the knowledge of surface tension of the studied amorphous material. In this case, a combination of nanoindentation measurements providing the near-surface viscosity with the measurements of surface structures flattening allows obtaining also the data of surface tension in the studied amorphous materials in the vicinity of glass-transition temperature.
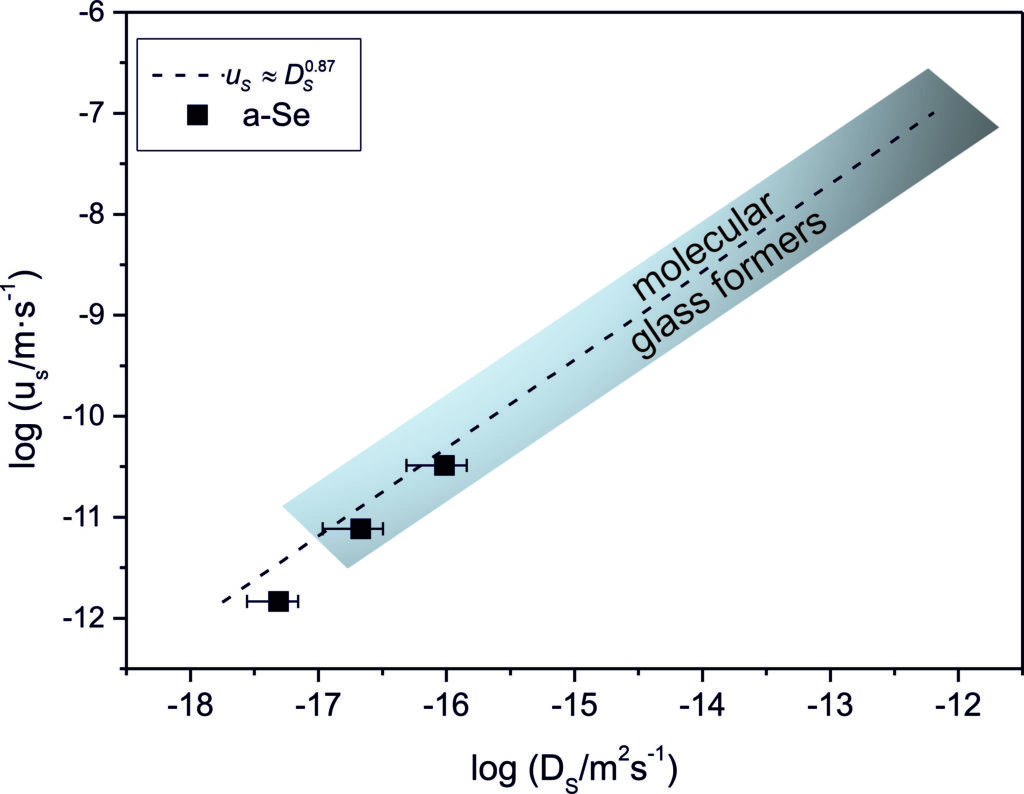
The above-described experiments are very important in the study of the surface properties of amorphous materials, especially concerning surface crystal growth. Recent studies on surface crystal growth indicate that there is a significant decoupling between the crystal growth rate and the viscosity describing the structural unit’s mobility according to the Stokes-Einstein relation generally used to substitute diffusion coefficients by viscosity in the contemporary crystal growth theories. Therefore, knowledge of surface diffusion coefficients would be of great importance in the surface crystal growth description. The extensive and detailed measurements on amorphous organic molecular substances, mostly published by groups of prof. L. Yu and prof. M.D. Ediger from the University of Wisconsin-Madison, show a strong correlation between the surface crystal growth rate (uS) and surface diffusion coefficient (DS): uS ≈ DS0.87. In collaboration with prof. Lian Yu and prof. Mark D. Ediger, we showed that similar dependence also holds for a-Se, as is shown in Fig. 3. Moreover, if the dependence of uS ≈ DS0.87 can be generalized for a broad range of materials, this could be of great importance, because from the knowledge of surface diffusion, the crystallization rate can be estimated – and vice versa – from surface crystal growth rates (often easier to measure) the surface diffusion coefficients can be assessed. Nevertheless, these statements need to be verified for more inorganic glass formers.