Non-crystalline materials lack the periodicity of crystalline substances. Structurally they resemble metastable supercooled liquids but behave mechanically like solids. They are by definition prepared by cooling a viscous glass-forming liquid fast enough to avoid crystallization. This way of preparation is known for millennia and it is used for fabrication of conventional glassy products such as windows panels, glass containers to more sophisticated materials such as bulk optical glasses for cameras and optical fibers that interconnect computer networks with recording devices, transmitting and finally bringing external world to our homes.
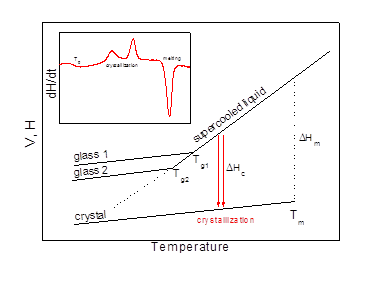
If the cooling through the melting temperature range is fast enough to avoid the nucleation and crystal growth, a supercooled liquid state is attained. The specific volume and other thermodynamic properties of the supercooled liquid can be extrapolated from the properties of the liquid above Tm. As a supercooled liquid is further cooled to lower temperatures, its viscosity increases and the molecular motions are gradually restricted. At sufficiently low temperatures, the characteristic time for these molecular rearrangements becomes comparable to the experimental time scale and a glassy state is formed. The glass transition temperature Tg depends on the cooling rate, being higher for faster cooling. As a consequence, there is not a single glassy state and the properties of the glass depend upon how it was obtained. There are more exotic ways how to prepare even a broader class of amorphous materials that lack the periodicity typical for crystals, such as ion bombardment, sputtering, fast vapor condensation, mechanical amorphization or sol-gel route, etc. The resulting amorphous materials exhibit very similar properties as glassy materials.
On reheating, the crystallization process is observed in many amorphous and glassy materials. In fact the crystallization mostly takes place above Tg in a supercooled glass-forming liquid, therefore, being substantially influenced by its viscosity. This process can be followed directly by microscopy or indirectly by macroscopic methods, such as thermal analysis (DTA or DSC), X-ray diffraction, electrical conductivity measurements, etc. Microscopic techniques are laborious, time consuming and often also experimentally difficult. Therefore, it is not surprising that they are frequently replaced by macroscopic methods and especially by DTA or DSC. Both these methods can be used to measure any process during which a measurable enthalpy change occurs. Despite the high universality of these methods, obviously there are also some shortcomings. Two most important ones are related to the detection limit and instrumental time constant. The sensitivity of modern DSC instruments is very high. However, for very slow processes associated with rather sluggish heat evolution, the signal to noise ratio might be well below the detection limit or the uncertainty of baseline subtraction may cause substantial errors.
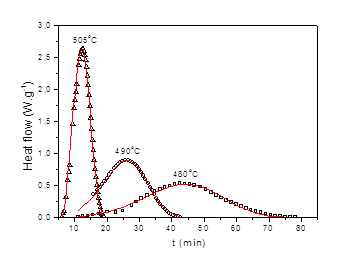
More than 1200 articles related to crystallization of glasses are published annually. This number is continuously growing being about 4 times larger than 20 years ago. Most of these papers are related to macroscopic methods such as DTA or DSC. Apart of this research line there is a significant volume of research work based on a classical microscopic study of nucleation and crystal growth.
Practical consequences of crystallization of amorphous and glassy materials are immense. Related problems can be found in geology and atmospheric physics. Another rapidly growing field is cryopreservation of biological materials. Crystallization is relevant also in industrial scale where very large-scale telescope mirrors are fabricated by controlled crystallization of a low thermal expansion glass. Opposite end of the industrial scale is related to emerging and very promising technology of computer phase change memory materials that are based on reversible crystallization of chalcogenide glasses. Extensive field of applications is related to optical recording media devices that are based on the same materials.
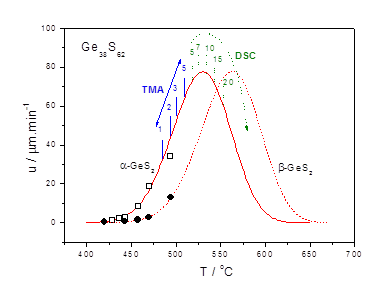
Our research attempts to understand complex processes of the crystallization of glasses and amorphous materials. We are combining the experimental results from thermal analysis, microscopy to get more detailed picture of crystal growth aiming to develop suitable models for description and prediction of crystallization extent under selected experimental conditions.
